Production Schedule
Does your MRP system have an integrated scheduling system that all users can access?
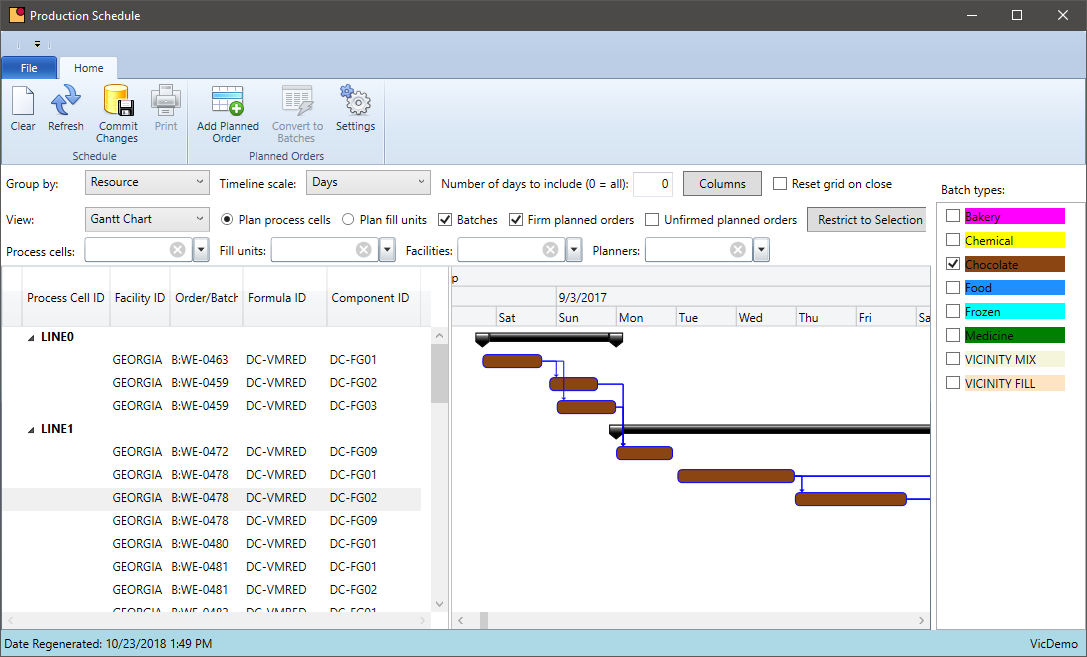
Chemical companies have several departments working together to produce their products: customer service, production, shipping, compliance, quality, and finance just to name a few. Keeping everyone working in the same direction requires a scheduling system that all users can access.
- Centralize scheduling data out of various spreadsheets and into a database everyone can share
- The schedule needs to be real time so as batches are completed, or new requirements are added, the schedule is updated automatically
- Include all data relevant to the schedule — forecasts, sales orders, inventory, inventory held by QC.
- Schedule on a defined calendar. Set rules for how soon the job can be scheduled
- Set aside time in the schedule for rush orders – exceptions will always happen so plan for them
- Group batches by type of formula where possible to reduce clean up or downtime
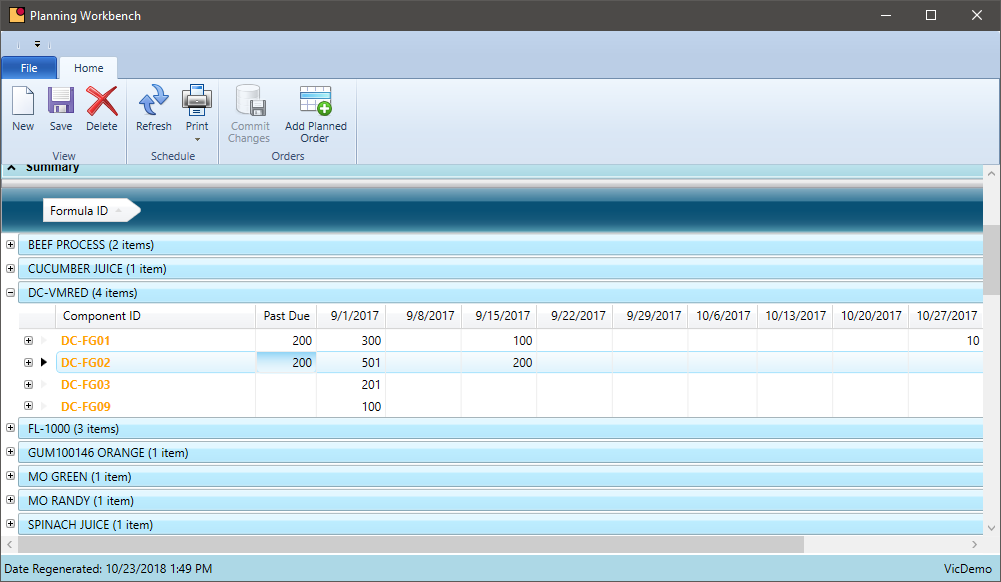
Group Scheduled Production by Formula
Do you ever package the same formula in multiple sizes? Does your system allow you to schedule items with the same formula together?
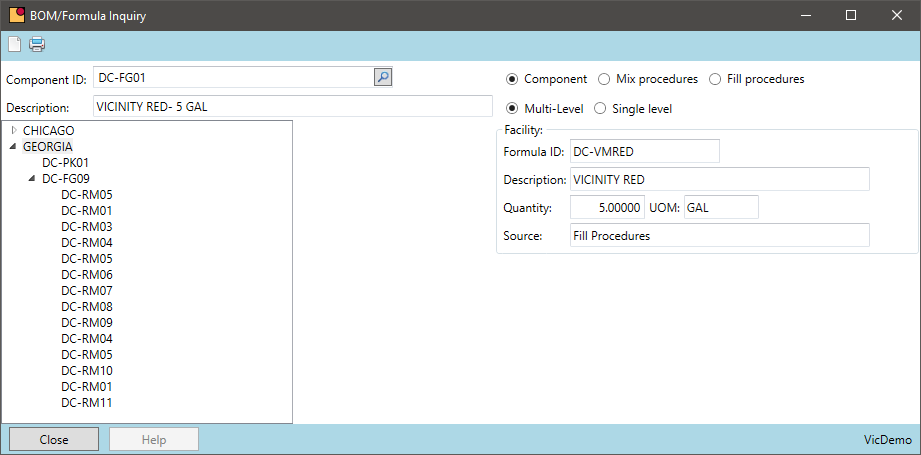
Chemical manufacturers often package the same formula in multiple packages sizes. The same formula may be packaged in 1, 5, or 55-gallon containers. Grouping this production by formula can reduce the number of batches in a period and provide other economies of scale.
- Include sales order demand as well as the forecasted demand to increase batch sizes where appropriate
- When using intermediates, aggregate the finished good demand to maximize the batch size resulting in fewer batches during a period
- Where possible, create one batch ticket that produced multiple end items. This will result in fewer batches in production and improved quality
- Leverage variable unit of measure conversions for finished goods to view output by volume or weight. This will help scale a batch to cover all items with ease
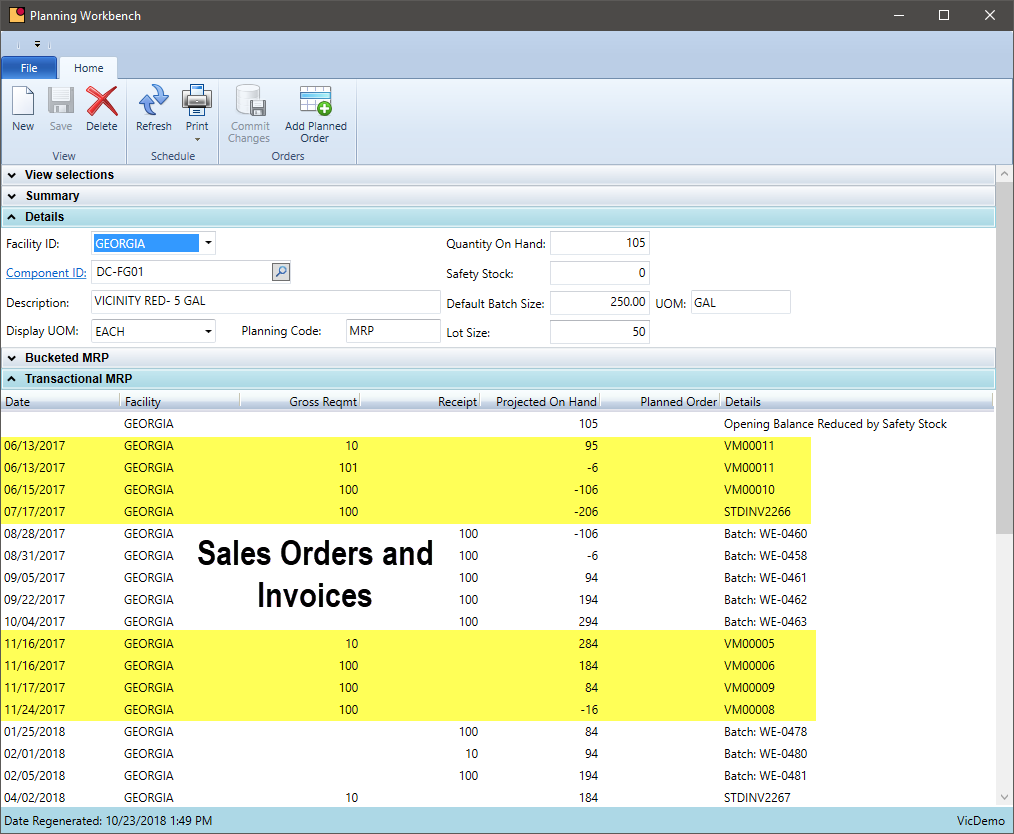
MRP – Sales Order Demand
How automated is your process to include existing sales orders in the production schedule?
The key to scheduling is to have the right product at the right time and not have too much on hand at any given time. Customer demand is not always predictable, but the ability to fulfill orders on-time and for the right quantity is critical. The production schedule and MRP software needs to include open sales orders as soon as practical and there needs to be a buffer for those customers who order with short lead times. The MRP system needs to accommodate this variable demand.
- Base sales forecasts on actual sales modified for seasonal and growth changes
- Integrated the forecasting tool into the planning system for a seamless flow of data during the scheduling process
- Join forecasts with sales orders and inventory levels to predict shortages before they happen
- Consolidate demand at a formula level and use a weight or volume to calculate an appropriate scheduled batch size
- Notify users when a new planned order is suggested inside the closest planning fence – give your production department as much heads up as possible
Work in Progress
Does your system account for the demand of finished goods as well as quantity on hand of intermediates and batches that started but not completed?
Chemical batches will vary in duration to complete. Some batches are simply mixtures and are completed quickly and other take reactions resulting in much longer production times. A scheduling and MRP system needs to account for batches that have begun but have not yet finished. It should also address partial completion of batches. Some finished good may be completed, but the remainder could be packaged later. Flexibility is key.
- Account for raw materials required for scheduled, in-process, and partially completed batches. Materials may be used, but finished goods may not be produced
- Allocate inventory to sales orders if there is something specific about a specific lot needed for a customer
- Record usage of raw materials as soon as practical and production of finished goods as soon as the inventory leaves production. Barcoding could help with this data collection effort
- Review open batch lists regularly to ensure all production has been recorded on a timely basis.
- Record actual usage and actual output. Do not backflush raw material usage based on finished good produced
Communicate the Schedule
How is the schedule communicated to departments? Is it printed? What happens when the schedule changes?
Changes in the schedule are inevitable. Once a change is approved, make sure to communicate the change in a way the department can accept. Sometimes printing is the proper way to communicate. If that is the case, include the printed date and time and set a time in the day when the schedule is updated.
- Where possible utilize a digital board to communicate the schedule. The visual calendar in VicinityChem works nicely for this
- Sort the schedule by production line and priority. Highlight rush orders
- Identify scheduling conflicts or anticipated quick changeovers. Giving that heads up to production is appreciated
- Keep users in the system as much as possible. This reduces the likelihood of data errors and miscommunication
- Adjust the user interface to accommodate the user where possible. Make the system easy for them to enter results
- Automate notification to users when raw material inventory falls into a critical stage. They may want to count inventory before staging the remaining items in the batch
Rejected Lots or Rework
How is a problem with a batch reported in the system? Can a lot be put on quarantine and not be used for scheduling or production but still be in inventory?
In a high-velocity chemical facility, a delay in quality can slow the entire system. Notifying production when a lot passes or fails quality control, as well as corrective actions, as needed on a timely basis.
- Notify users electronically when QC fails a lot or prescribes corrective action. Provide batch information, materials to be added or further processing required.
- Notify users electronically when QC approves a lot. This will reduce the time to get the product to packaging
- Enter corrective action taken as soon as practical. This will keep raw material inventory accurate as well as communicate to the quality lab that another test is forthcoming
- Quarantine lots as soon as quality has made the determination. Transferring the inventory to a quarantine location will provide visibility to the schedule and other users that the lot will not be available.
- Quarantine lots should be placed on hand, but the system should reduce them from available inventory
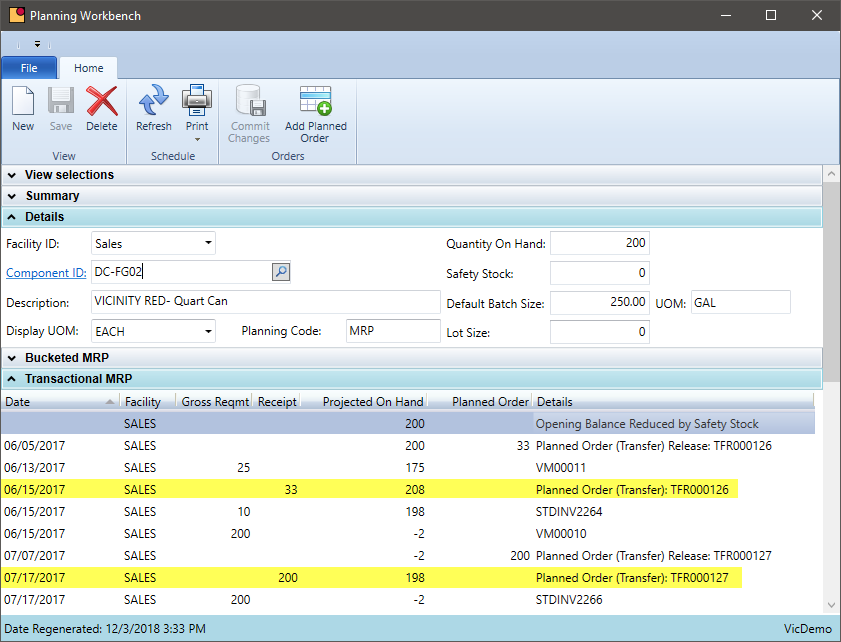
Warehouse Transfers in MRP
Does your MRP system suggest transfers from one warehouse to another or is this a manual process?
Many chemical manufacturers maintain inventory at multiple warehouses spread out geographically or are near key customers. Keeping the right level of inventory at various locations can be a time consuming and expensive task. VicinityChem leverages the MRP software functionality to suggest transfers from warehouse locations that are making the product to those warehouses selling the product. These suggestions are planned orders and the logistics personnel can be alerted when a transfer is required.
- Notify users electronically when inventory in one location is not sufficient to supply orders or fall below a minimum stock level
- Generate planned transfer orders to assist logistics with the transfer but also place demand on the production location
- Include transfers in process to eliminate duplicate transfers
- Generate bills of lading for transfers
- Maintain lot traceability from warehouse to warehouse to customer