Operations for batch chemical processes
Learn how VicinityChem can help your operations run more efficiently across all departments with centralized information, specifications and formula management for your batch chemical processes.
Integrated Data
Do you have data being kept in different systems or spreadsheets across multiple departments?
Chemical manufacturers need up to date and accurate information to fulfill orders successfully and comply with various hazardous reporting requirements.
- Maintain all compounding and filling batch tickets in an integrated database
- Chemical and material used on a batch is posted automatically with our chemical inventory software
- Apply labor and overhead to each batch to provide a clear view of the cost of production by weight or volume of chemicals produced
- Make it easier to get actual data into the system. Employees spending excessive time recording data is inefficient and often dangerous in a chemical environment
- Enter information one time into one system to increase efficiency
- Timely and accurate data will help chemical companies better calculate their true cost of production
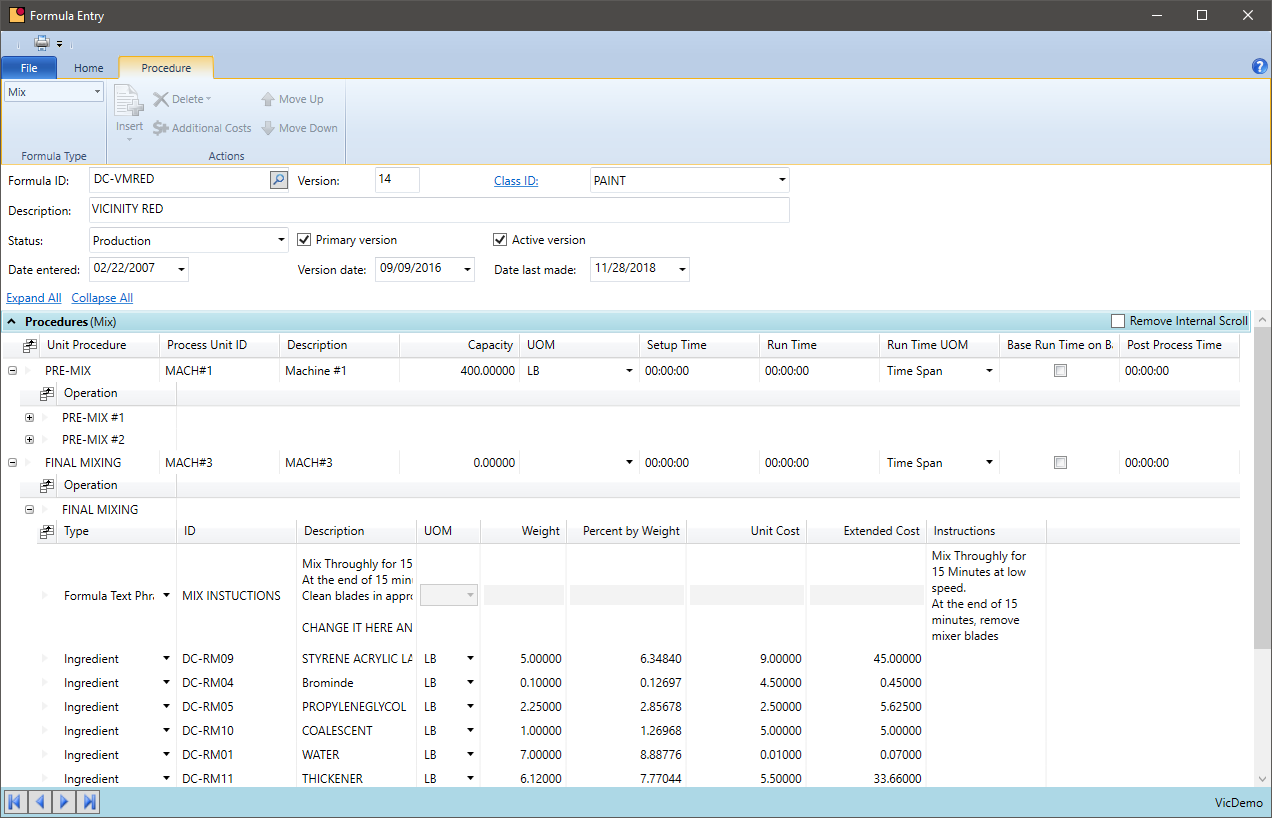
Formula Management
Do you have batch formulas in multiple sources like excel for formula management and batch tickets and inventory in another system?
Supplier changes, material availability, or process changes often cause changes to multiple formulations and finished goods. It is important to communicate those changes efficiently to various departments of a chemical manufacturer.
- Centralize all formulas (BOM) integrated into a common chemical inventory software system
- Base a batch ticket on a master formula, not a finished good, and allow version control to ensure the most accurate formula version is being used
- The formulas created in R&D should be the same in batches as well as inventory control
- Assist the production process by defining the mixing or compounding vessels and stages of production inside the formula, so there is one source of how the chemical is processed
- Leverage the ability VicinityChem for batch sizing and to convert between weight, volume, eaches, and metric to simplify data collection and entry
- Routinely review batch yields in actual production and update the master formula. This will help MRP and scheduling better define the material requirements. Integrated chemical inventory software will speed up the ordering process
- Include labor and overhead into the master formula, so subsequent batches are applying the most up to date standards
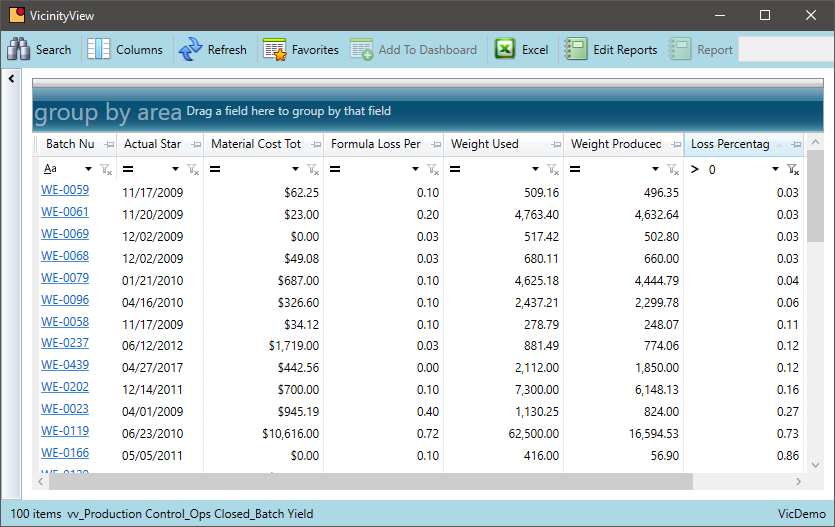
Batch Loss
Are you looking at how much material you put into the batch compared to what you got out? Don’t forget to account for quality adds or re-work applied.
Chemical companies need to keep an eye on changing batch losses. A change in actual yield will affect production costs, inventory levels, and the ability to fulfill sales orders based on customer requests. Identifying these shifts in formulas that are experiencing changes is key to solving the problems before they become significant.
- Measure losses on a batch by batch basis by comparing actual material input to the actual produced output
- Review losses over time as a routine part of the operating cycle
- Highlight formulas where the real batch loss significantly varies from the expected formula loss
- Configure the system to alert users when a batch is closed with a significantly different yield than the original batch sizing
- Work with multiple departments to identify the cause and take corrective action for the change in yield
- Yield changes can signal preventative maintenance requirements on equipment. Make sure to involve the maintenance department where necessary.
- Document unusual occurrences during the batch process. These could be a one-time event, or they could be caused by other factors in the chemical manufacturing process
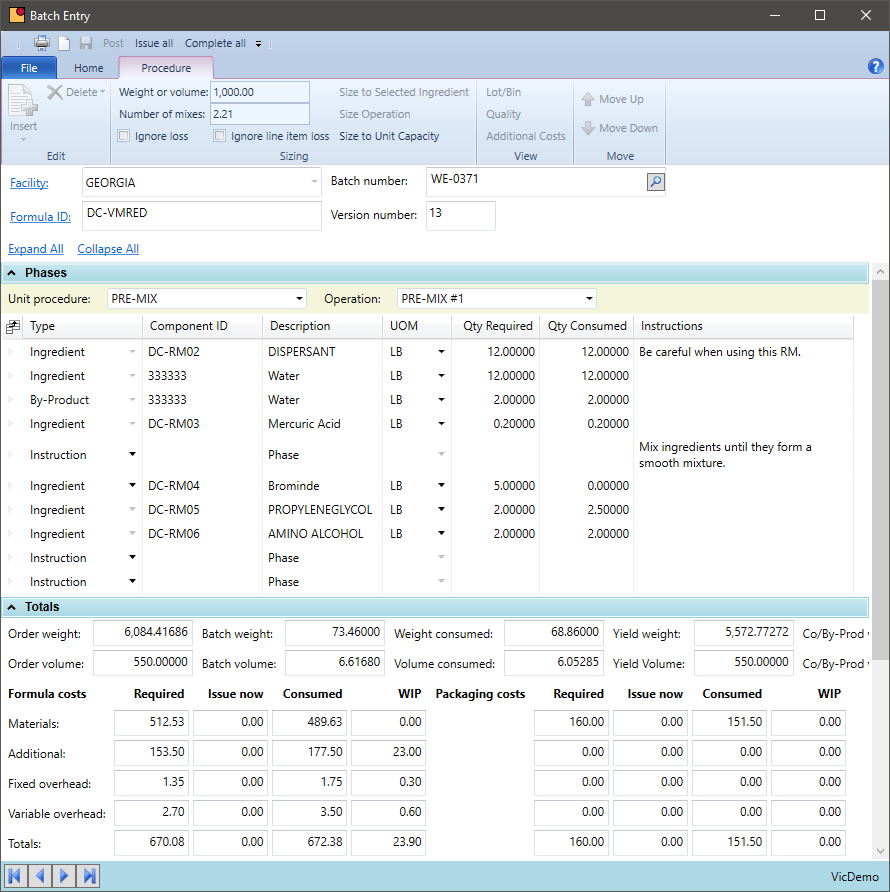
Actual vs. Theoretical Usage
Does your staff record actual raw material usage and actual production? Or is everything backflushed from produced chemicals?
A formula is a guideline for production to follow. In some industries, this is very rigid and in others there is room for variation. A system is needed that will provide the structure required but when a variation is called for, the system will accept it. It is all about telling the system what really happened in the chemical process.
- Record actual versus planned raw-material for each batch – this includes QC additions and the introduction of re-work material
- Note when different equipment is used or more or less labor is applied
- Identify and report when batches use a significantly different quantity of materials as this can highlight a data entry or processing error
- As actual inventory usage is recorded, inventory will become more accurate, and users will be able to rely on the inventory records resulting in fewer expedited orders and delays in production
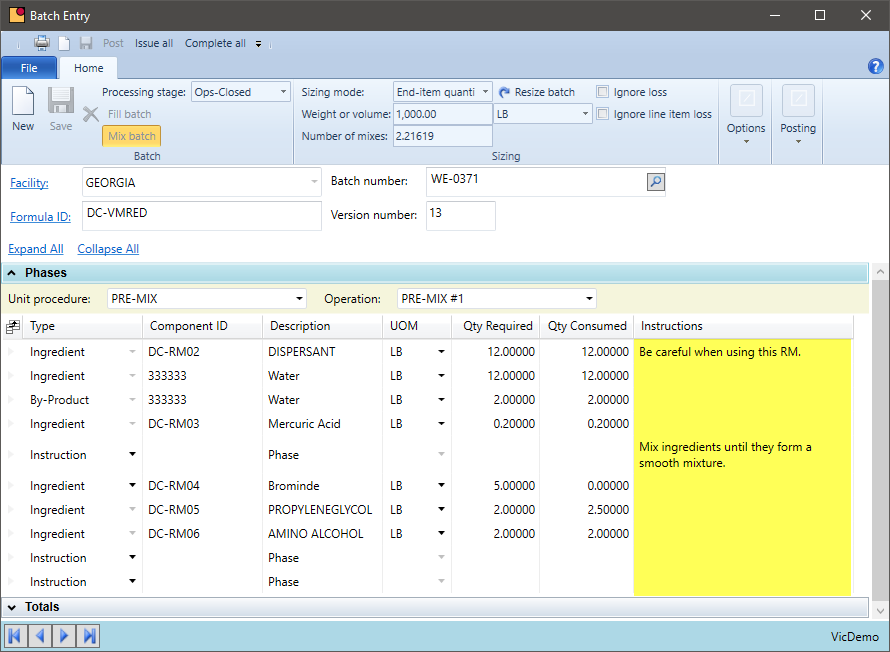
Processing Instructions
Are your processing instructions part of your centralized formula, or are they kept in a separate system?
By including your processing steps, set-up instructions, and operator instructions, you are able to centralize your data, reducing the data-entry requirements and increasing data integrity.
- Assign instructions, machine settings, run times, and other attributes at the formula-version level. That way, all batches get consistent information
- Bring all formulas and related production data out of Excel or custom databases into a central location for ease of management and reporting
- All batches should originate from this data source to reduce the likelihood of obsolete information getting into production
Print OSHA and PPE Disclosures to Material Handlers
Does material handling and OSHA safety information print on the batch ticket?
Provide a central location for material handling and personal protective equipment (PPE inventory) notifications for all chemicals and ingredients used in a formula. When a batch is created from that formula, the hazard information can be presented. This can provide consistency and hazard communication for all batches.
Communicate Quality Control Tests to Be Performed
Do prescribed quality control tests or observations print on the batch ticket that goes into production?
Defining QC tests or processing observations on the master formula or against a produced item allows for a central data location. When a batch is created, these specifications are automatically included on the batch ticket. This provides a standard the batch handlers can rely on.
- Define QC tests to the master formula and produced items in a central database and outside of Excel
- Provide test specifications so the operator can determine if the batch is out of specification so corrective action can be taken
- When results are entered against a batch, notify appropriate users when the batch is out of specification
- Some tests are done at certain stages of mixing – apply the criteria at the stage of production required to define the processing steps of the mix or compound better
- Dashboards can provide users with a quick view into QC data trends of ongoing batch QC data. Corrective action can be taken quickly
Inventory Control
Can you go to your current system and rely on the quantity it says you have on hand right now?
Inventory transactions must be timely, accurate and complete. If any of those are missing, the data will not be reliable and users will build workarounds to get the information they need.
- Record inventory transactions as soon as practical using a user-definable user interface to increase the efficiency of data entry
- Cycle count high-dollar, high-velocity chemicals
- Minimum stock levels are good to use for chemicals with long shelf lives and are not used frequently
- Where possible, record transactions as they occur. This will give insight into how many times the same chemical is being added to the batch
- Use a single batch ticket to put multiple chemical storage sizes on hand
- Leverage units of measure to aide the material handler from having to convert units from volume to weight
- Streamline chemicals by finding all formulas that use the same materials with the Where-Used Component report
- Record production as soon as practical. This will put inventory on hand on a timelier basis and allow the warehouse to pick lot track items sooner than waiting till the end of the batch
- Deploy barcode data collection software and hardware to reduce the latency of inventory getting into the system.