Review Quality Control Data for Formula Accuracy
Do you ever look at the number of quality adjustments made on batches? It can give valuable feedback on the changes needed for formulas.
Every time a batch goes back through QC, it costs the company money. By tracking the number of times a batch goes through QC and what change are made, formulas can be identified for an R&D review, saving time on future batches.
- Track QC tests that occur on a batch in a timely fashion
- Routinely review the number of tests performed by formula to ensure all and only those tests needed are defined for the formula
- Review the number of batch additions made by formula over a period. Investigate those formulas with a higher than average frequency of quality additions.
Certificates of Analysis (COA)
Can your system create or store a certificate of analysis from your vendors or produced by your company? Are these generated from a central location pulling data from formulas, chemicals, and quality results?
Moving the printing of the certificate of analysis from the QA lab to the shipping department can save valuable resources for a company.
- Scan and save vendor COAs against a raw material lot so all users with access can review the data as needed
- COAs can be generated for all lots shipping on a sales order, all lots produced in a day or all lots of chemicals produced
- Define target ranges and high and low specifications by test by formula or compound
- Notify users when quality results for a received raw material lot do not comply with the material specifications
- Restrict lots that have not been approved for production
- Generate customer specific COAs based on customer service requirements
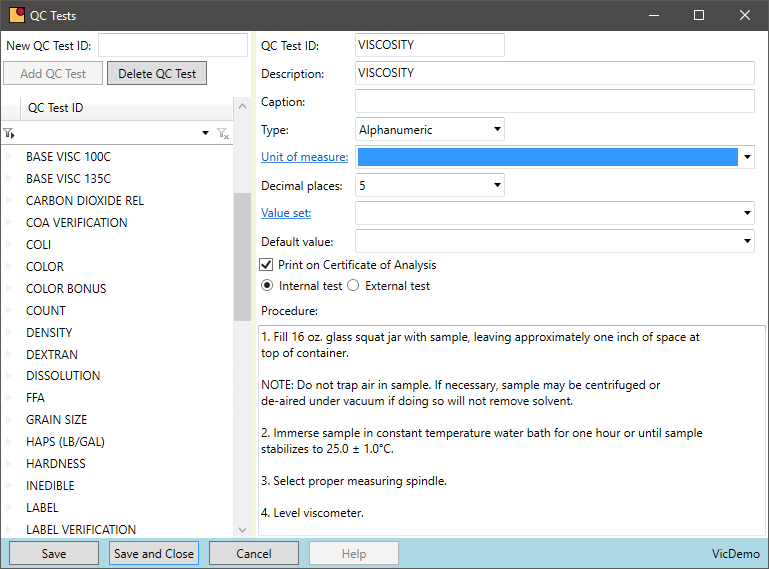
Standardized Quality Control Tests
Is there a central location for all QC tests to perform for a purchased or produced chemical?
The chemical industry is required to track a significant amount of data. If a non-conforming lot is added to a batch, it could be very costly to fix the problem. Getting it right the first time is a must.
- Making all the QC tests and specifications available on a printed batch ticket or on an inquiry screen is essential to streamline the chemical manufacturing process
- The master formula is the template for all quality data on a batch. When a test is added or changed on a formula, all subsequent batches inherit those changes
- Defining target results and high/low ranges on the formula supports the QC result data entry and speeds processing for the lab technician
- VicinityChem has a list of standard tests to be used out of the box. An unlimited number of tests can be added, and the standard tests can be removed
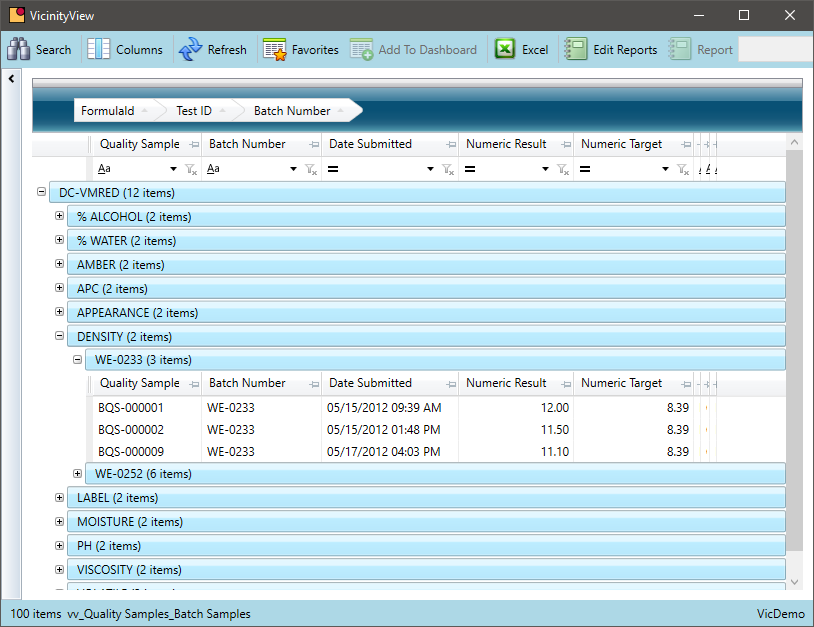
Query Quality Control Test Results
Is it easy to view quality results and connect it to other data in your system?
Reviewing QC test results are essential to a chemical manufacturer. Being able to associate that quality data with data from other departments add even more value to the information.
- Query QC results over time by formula or chemical; batch or lot
- Leverage the lot trace capability to tie QC data for raw material to QC data for a finished good
- Research all quality data for a specific lot or batch to identify patterns over time
- Summarize results to help establish new formula standards or identify formulas that require further R&D review
- Utilize any dashboard tool to connect to QC data and review trends using intuitive graphs and charts maintained by the user
Quality Scheduling
Can your system help you schedule the lab workload?
To run an efficient chemical quality lab, it is important to schedule for busy times and adjust for slower times. Utilizing the production schedule and integrated QC tests assigned to the formula or chemical can assist the quality lab manager in scheduling technicians.
- Review the production schedule in real time
- View tests required based on the schedule using a user-definable query tool
- Navigate to quality result entry directly from the schedule. This speeds up processing and allows the user to validate they are processing the correct batch results
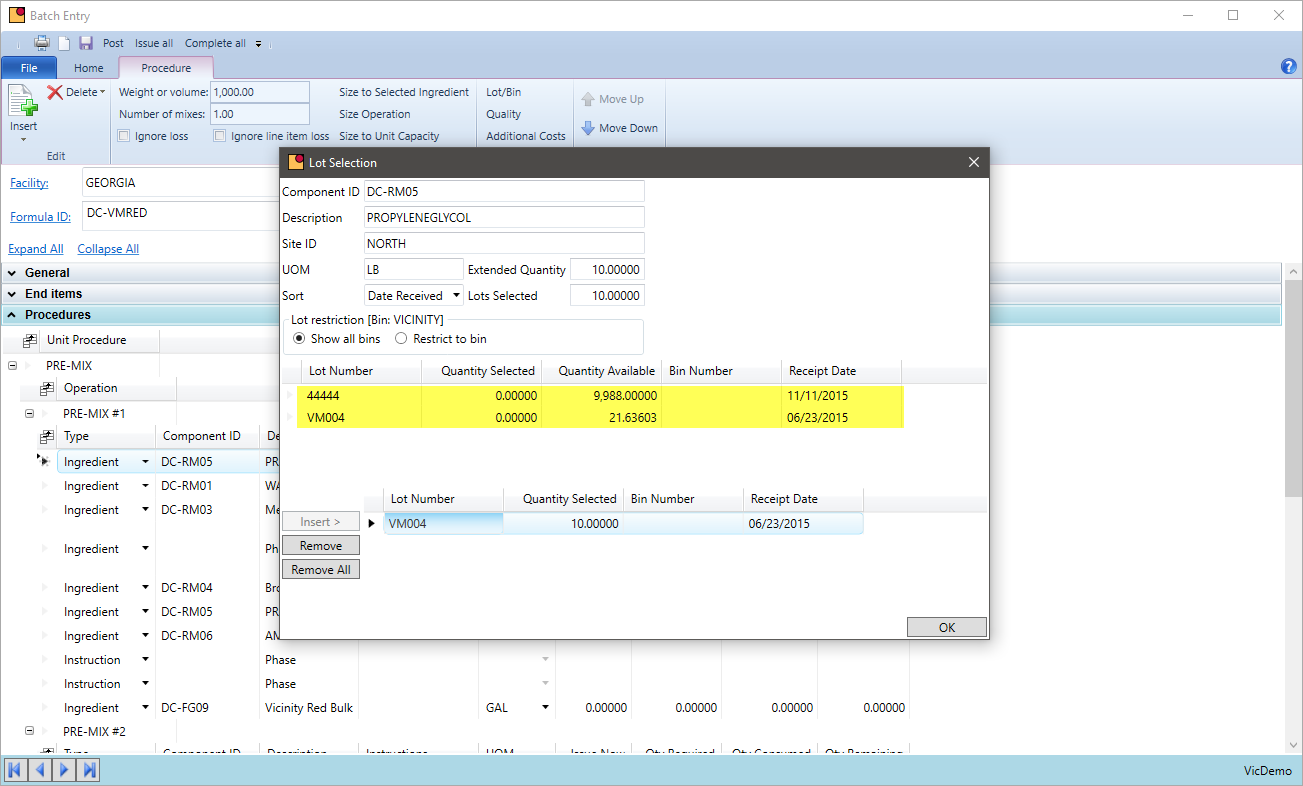
Lot Approval
How do you currently ensure only approved lots are used or shipped? Can lot tracking be turned on for only specific items?
VicinityChem supports the quarantine of unapproved lots as well as releasing them for production once approved by quality. During that time, the chemicals are on hand but not available to be used by production nor are they seen by MRP. The same is true for intermediates and packaged goods. Shipping can be restricted to only ship lots that have been released from quality hold. This lot tracking streamlines the approval process as well as adds a level of security that a lot will not be used or sold until it is released.
- Approve or reject lots during purchase receiving or place on quarantine for quality to release
- Lot track certain items and restrict lots to be used in production. Allow only those that have passed QC.
- Restrict by chemical lots that can be shipped or restricted from picking
- Inquire lots requiring QC inspection so quality can test and approve or resolve
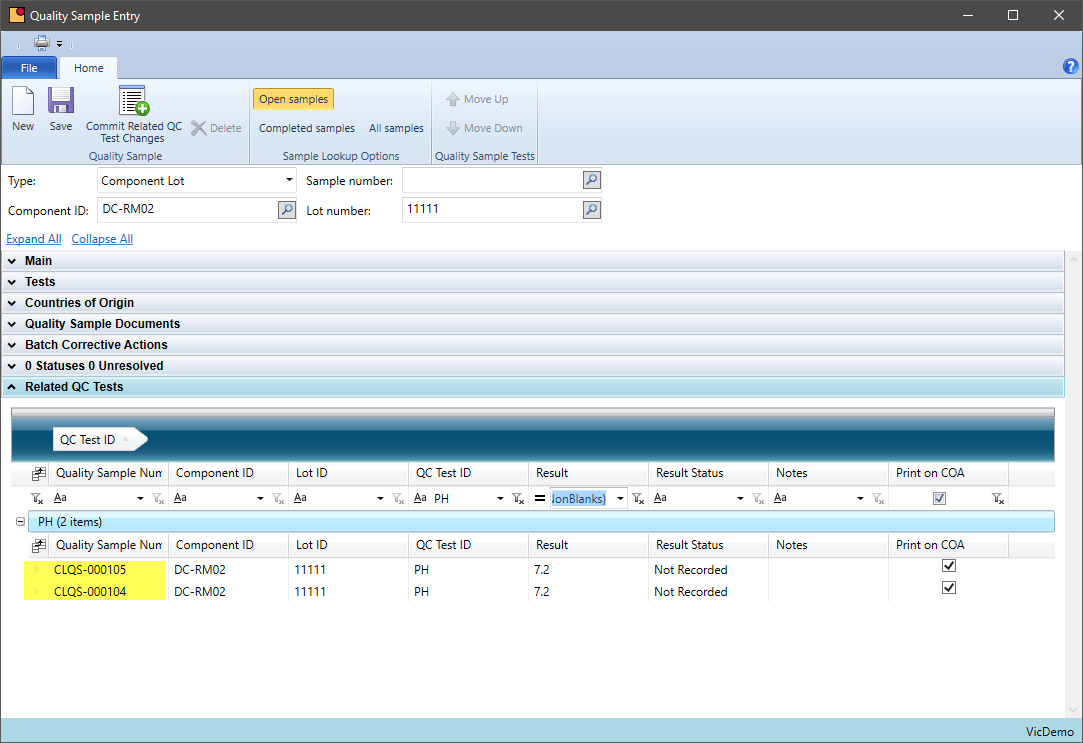
Quality Additions
Can you add chemicals not prescribed on the original batch ticket?
Some chemical companies need to add additional chemicals to bring a production batch into specification. These chemicals may be part of the formula or may be a chemical that is being introduced just for this batch. VicinityChem allows a user to add a chemical to a batch, track that it was caused by a quality requirement, and review how often this is occurring by formula.
- Define quality additions for a batch within quality sample entry
- Inquire how often quality additions are made for a formula
- Add any chemical to a batch. It did not have to be a part of the original formula - including re-work
- Apply costs of chemicals added to the batch for more accurate production cost tracking